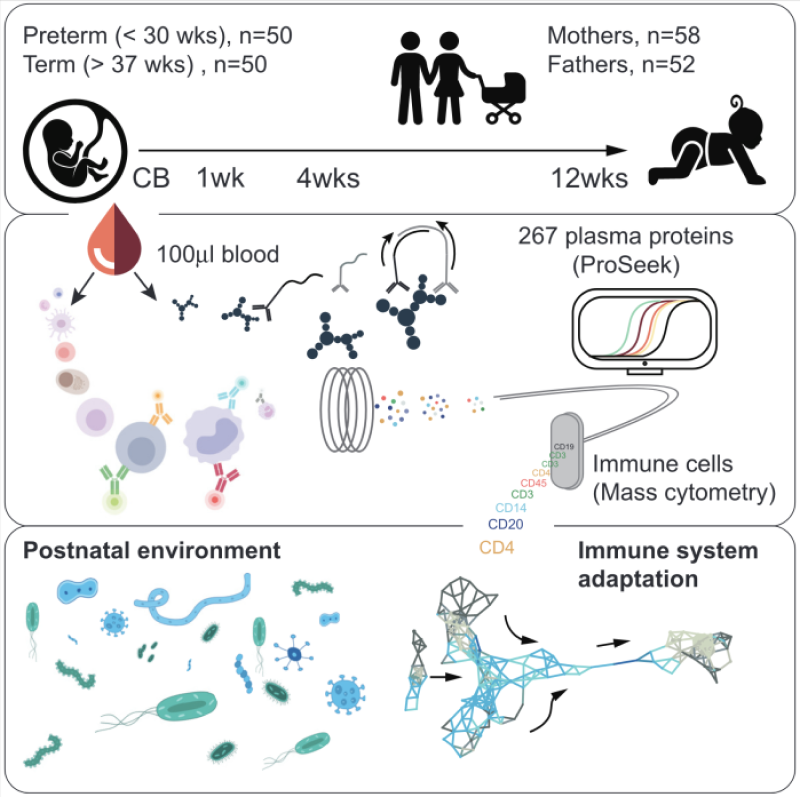
Born Immune is an ongoing study that was initiated almost 10 years ago. It was set up to follow the physiological development of children born at term (> 37 weeks of gestational age) and those born very preterm (< 30 weeks of gestational age). Collecting blood samples from neonates born as early as 24 weeks after conception is incredibly challenging, as they require intensive care and have very low blood volumes, meaning that only around 100 microliters of blood can be collected at a single sampling occasion. Anna Karin Bernhardsson is a research nurse and responsible for organizing the sample collection of the Born Immune study.
"This study involves clinical sampling in a range of hectic clinical situations. The simplicity and flexibility of the Cytodelics Whole Blood Stabilizer allowed us to quickly introduce a clinical sampling routine at the maternity ward and the neonatal intensive care unit." - Anna Karin Bernhardsson
The Born Immune study uses the Cytodelics Whole Blood Stabilizer to preserve minute blood samples for later analysis. In an initial paper by Olin et al., they presented longitudinal data on the immune phenotype of 100 newborn babies. They used mass cytometry to investigate changes in white blood cell phenotypes during the first three months of life and how this development differs between term and preterm neonates. They discovered that while children born term and preterm are initially very different immunologically, they became almost indistinguishable after a few months.
"Work at the neonatal intensive care unit can be very stressful, and our staff often have to leave in the middle of a sampling procedue to deal with some clinical emergency. Cytodelics products are insensitive to deviations in sample volume, storage time, and temperature, which is really helpful in these situations." - Anna Karin Bernhardsson
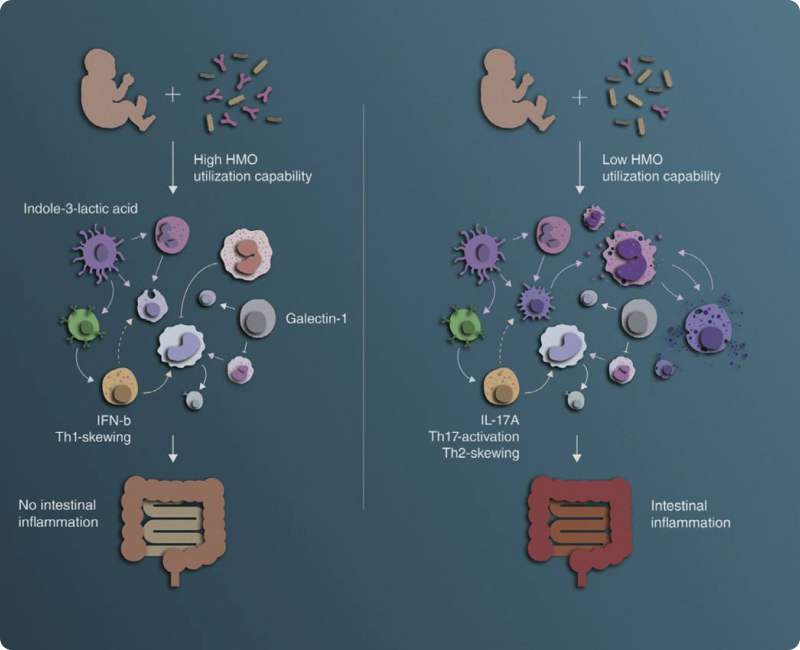
In a follow-up study including 208 infants, the authors investigated how the immunological changes observed in the first study related to parallel changes in the gut biome. They observed that certain immunological changes, such as the levels of monocytes and the cytokine IL-17 in the blood, were related to the levels of Bifidobacteria species in the gut. In an intervention study, they demonstrated that adding a Bifidobacteria-containing supplement led to decreased intestinal inflammation.
"We still occasionally perform Ficoll titrations to isolate PBMCs. In these cases, you're tied up for two hours and unable to help out with clinical work. Collecting a sample with Cytodelics buffer only takes seconds, and as there's no lower limit for the volume of blood you can collect, it's been possible to retreive cells from samples that we would otherwise have thrown away. It's really been a game-changer for us!" - Anna Karin Bernhardsson